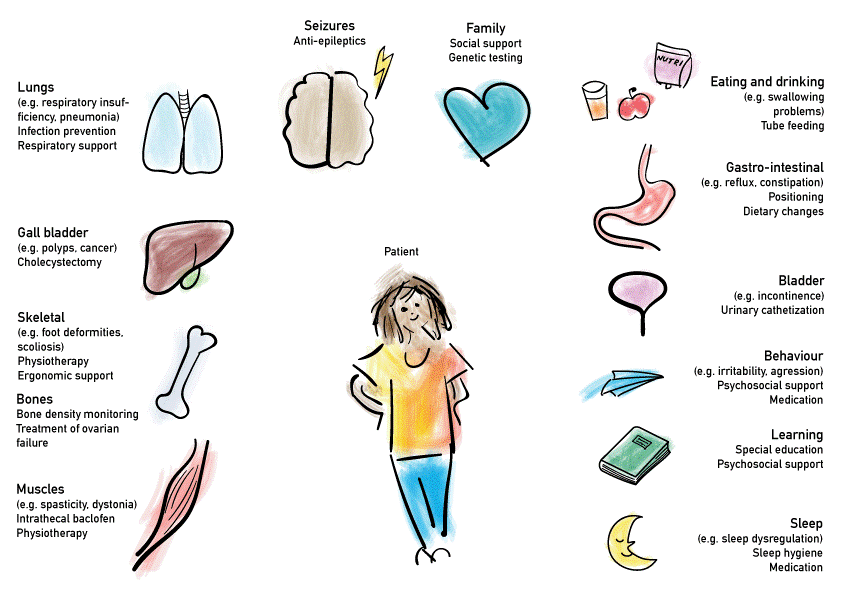
Supportive care
© 2021 mldinitiative
Symptomatic treatment is an important aspect of disease management in MLD. Supportive care plays a big role in patients that are not suitable for causal therapy as well as patients who receive causal therapy. Some important elements of supportive care are summarised below.
• Laparoscopic cholecystectomy
• Tube feeding
• Anti-spastic treatment, including intrathecal Baclofen
• Anti-epileptic drugs in case of epilepsy
• Social and psychological support
Currently ongoing trials
- Gene therapy in late-juvenile MLD: This therapy is already authorised for late-infantile and early-juvenile patients in Europe. Current trial investigates the safety and efficacy in late-juvenile patients. The trial is executed in Ospedale San Raffaele in Milan. The company is Orchard Therapeutics. Detailed information can be found on ClinicalTrials.Gov
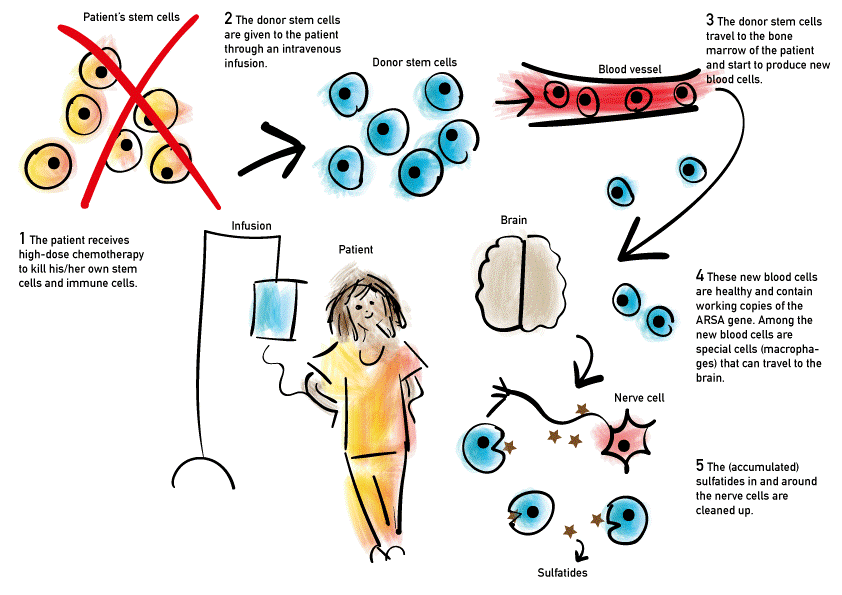
Hematopoietic stem cell transplantation
© 2021 mldinitiative
Treatment with an allogeneic hematopoietic stem cell transplantation (HSCT) provides a clinical and survival benefit for pre- and early-symptomatic patients with juvenile and adult MLD. Currently, this is the standard treatment in patients with MLD. After myeloablative conditioning, usually with fludarabine and pharmacokinetic-guided busulfan dosing, the patient receives a transfusion with hematopoietic stem cells derived from bone marrow, peripheral blood, or umbilical cord blood of a healthy donor. These donor cells migrate to various organ systems, including the central nervous system, and replace the affected cells to provide continuous production of ASA. However, the replacement of ASA deficient host cells in the central nervous system is relatively slow, resulting in a delay estimated at 12–24 months until the disease stabilises. Besides that, HSCT is an invasive treatment and often results in initial deterioration of symptoms. This makes HSCT unsuitable for MLD patients with rapid progression of the disease, including patients with (pre-symptomatic) late-infantile MLD and patients with advanced juvenile or adult MLD.
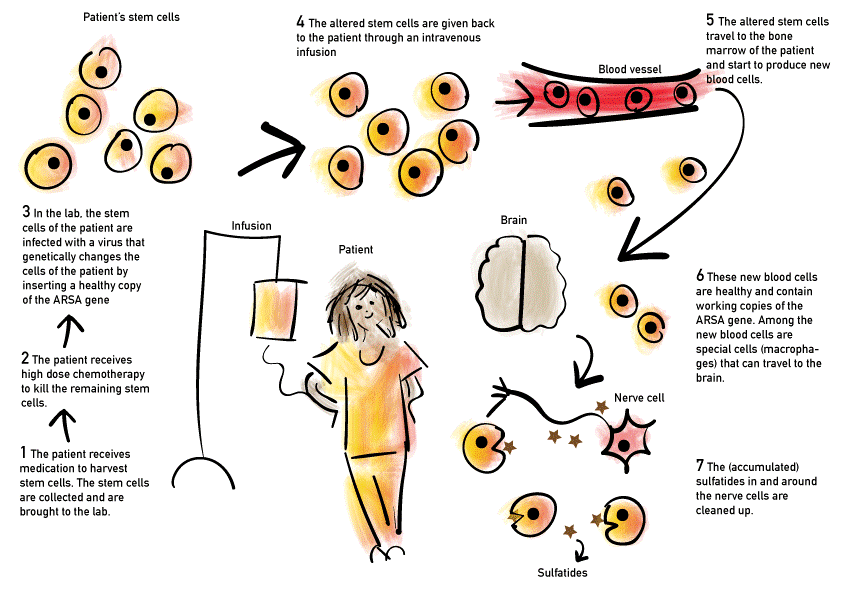
Gene therapy
© 2021 mldinitiative
Autologous HSCT-based gene therapy is a treatment that results in good functional outcomes for pre- and early-symptomatic patients with late-infantile MLD. In this treatment, autologous hematopoietic stem cells are transduced with a lentiviral vector containing a healthy copy of the ARSA gene. Depending on the vector’s promoter, the ARSA gene can be expressed at much higher levels than those normally present in hematopoietic stem cells and their offspring, thereby providing a faster and greater clinical benefit than allogeneic HSCT. Clinical trials showed robust evidence of safe, stable transgene expression and improved clinical outcomes in both late-infantile and juvenile MLD patients younger than the age of 7 years. Nevertheless, long-term treatment effects and safety outcomes have yet to be determined (i.e., beyond the current 15 years of hindsight), and MLD patients with later disease onset are not yet eligible for this treatment.
Libmeldy™
December 2020 the medicinal product for gene therapy with the name Libmeldy received a marketing authorisation from the European Medicines Agency.
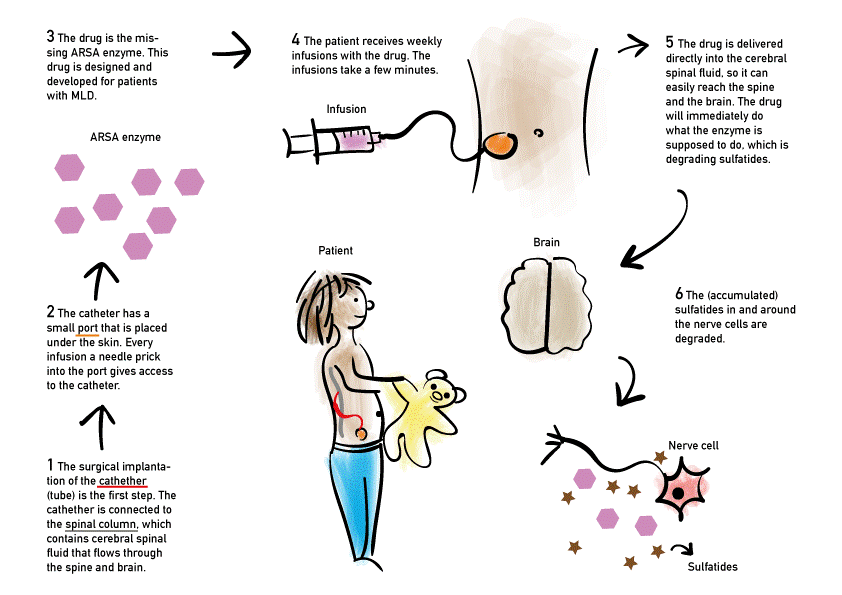
Intrathecal enzyme replacement therapy
© 2021 mldinitiative
Intrathecal administration of recombinant human ASA for symptomatic late-infantile patients is currently investigated in a clinical trial. It is supposed to degrade sulfatides. The first results of a phase 1/2 trial showed that this treatment is safe in the short-term, but treatment effects still have to be demonstrated. The trial is not actively recruiting at the moment [updated: 12-2022].
